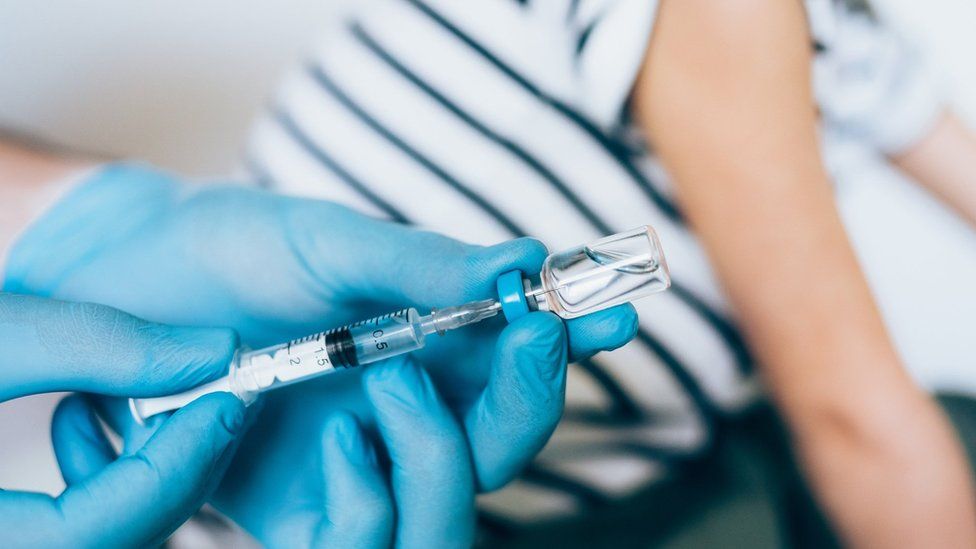
Covid Oxford-AstraZeneca vaccine to be tested on children
A new trial is to test how well the Oxford-AstraZeneca coronavirus vaccine works in children.
Some 300 volunteers will take part, with the first vaccinations in the trial taking place later in February.
Researchers will assess whether the jab produces a strong immune response in children aged between six and 17.
The vaccine is one of two being used to protect against serious illness and death from Covid in the UK, along with the Pfizer-BioNTech jab.
As many as 240 children will receive the vaccine – and the others a control meningitis jab – when the trial gets under way.
Volunteers who live near one of the four study sites – the University of Oxford, St George’s University Hospital, London, University Hospital Southampton and Bristol Royal Hospital for Children – are being asked to sign up.
Those interested in taking part must complete a short questionnaire.
Andrew Pollard, professor of paediatric infection and immunity, and chief investigator on the Oxford vaccine trial, noted that most children were relatively unaffected by Covid and were unlikely to become unwell with the virus.
But he said it was important to establish the safety and immune response to the vaccine in children and young people as some children might benefit from vaccination.
There are currently no plans for children to be vaccinated with the Oxford-Astrazeneca jab in the UK, as it has only been authorised to prevent Covid in people aged 18 or over.
The Pfizer-BioNTech jab is only authorised in those aged over 16. The vaccine priority list also excludes anyone under the age of 16, even the clinically extremely vulnerable.
Earlier this week England’s deputy chief medical officer, Prof Jonathan Van-Tam, told ITV News several trials were under way to develop vaccines that were safe and effective in children, saying it was possible there would be some licensed children’s vaccines by the end of the year.
The University of Oxford said its was the first trial of a Covid vaccine in the six to 17 age group. It said other trials had begun but only measuring efficacy in those aged 16 and 17.
The Royal College of Paediatrics and Child Health says there is evidence Covid-19 can cause death and severe illness in children but this is rare.
It said: “In children, the evidence is now clear that Covid-19 is associated with a considerably lower burden of morbidity and mortality compared to that seen in the elderly.
“There is also some evidence that children may be less likely to acquire the infection. The role of children in transmission, once they have acquired the infection, is unclear, although there is no clear evidence that they are any more infectious than adults.”
A third vaccine, from Moderna, has been approved for use in the UK but supplies are not expected to arrive until spring.